Dehydration Synthesis Reactions Are Used in Forming Which Compounds
Dehydration reactions are also involved in the production of many polymers. Learn vocabulary terms and more with flashcards games and other study tools.
This Is A Polypeptide The Polymer Of A Protein It Is Made When Amino Acids Link Together Using Peptide Bonds Peptide Bond Protein Biology Amino Acids
Many reactions involving dehydration synthesis are associated with the formation of biological polymers where the addition of each monomer is accompanied by the elimination of one molecule of water.
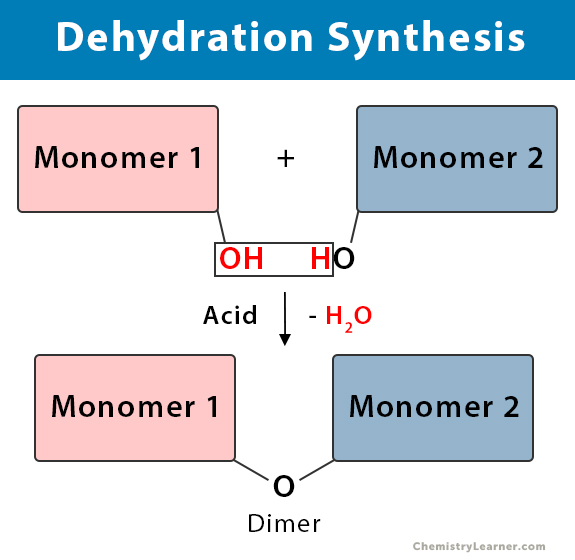
. Use the dehydration synthesis. Process that breaks apart polymers into separate monomers by adding water. Or compounds together following the removal of water forms polymers.
Main energy source for most types of cells. 2 CH 3 COOH CH 3 CO 2 O H 2 O. Dehydration synthesis is a key step in the formation of polymers large molecule chains formed by linking together multiple monomer units via dehydration of course.
A monosaccharide with the chemical formula C6H12O6. Cboth of the reactants. B only one of the reactants.
Dehydration reactions are also used to form important organic compounds such as ethers. Two alcohol molecules can undergo dehydration synthesis to form an intermediate ion that ultimately loses a. A molecule that can be bonded to other identical molecules to form a polymer.
CHO always found in this compound mostly carbon. Dehydration reactions are a subset of condensation reactions where two functional groups combine to form a covalent bond along with the release of a small molecule. A monosaccharide with the chemical formula C6H12O6.
In condensation reactions the atoms that make up a water molecule are derived from a oxygen. Dehydration synthesis occurs when there is a loss of water molecule for the formation of a larger molecule with the help of small reactants. A symbolic representation of an element or compound.
View the full answer. Many different biological or chemical processes utilize the dehydration synthesis reaction. Most of the dehydration synthesis that we see occur in nature forms a biological polymer where we get to see the addition of individual monomers along with the elimination of a single molecule of water.
Reactions that produce acid anhydrides are dehydration reactions. D Hydrolysis creates monomers and dehydration reactions destroy them. Organic Compounds Test Joseph 42 terms.
Carbon hydrogen oxygen nitrogen phosphous sulfur. DME production is based on methanol dehydration where two molecules of methanol are converted into DME releasing a molecule of water in the presence of a catalyst preferably γ -Al 2 O 3 modified with SiO 2 Jun et al 2002. Dehydration reaction is a reaction where molecules are joined by elimination of a water molecule.
These include molecules like proteins lipids fatty acids nucleic acids DNA. A carbohydrate made of two monosaccharides. Dehydration synthesis takes place when the monomers of organic compounds join together by a chemical reaction to make polymers.
For example acetic acid CH 3 COOH forms acetic anhydride CH 3 CO 2 O and water by the dehydration reaction. Hydrolysis its the opposite reaction of breaking up polymers and is. An organic molecule made up of carbon hydrogen and oxygen.
Triglycerides are formed when three OH groups of the the glycerol molecule react with the carboxylic acid of the three fatty acid by removal of three. HOW DOES THE DRAWING FOR DEHYDRATION SYNTHESIS LOOK. On the other hand just like dehydration.
An acid catalyst can be used during a dehydration synthesis reaction. Examples include the formation of disaccharides like sucrose from monosaccharides like glucose and alcohol condensation to make an ether product. Polymerization reactions are good examples of dehydration synthesis reaction in which monomer units condense together to form polymers.
E Dehydration reactions occur only in animals and hydrolysis reactions occur only in plants. Formation of disaccharides from monosaccharides in carbohydrates the formation of lipids with one glycerol and three molecules of fatty acids are examples of dehydration synthesis. Primary molecule used during cellular respiration reactions.
Yaripour et al 2005. Start studying Dehydration Synthesis Reaction Cards. The second example a biological process will involve the dehydration synthesis of glucose a monosaccharide to form a disaccharide sucrose.
In biology dehydration synthesis forms large polymers commonly referred to as biological macromolecules. Dehydration synthesis reaction picture. A chemical reaction in which monomers are binded together to form either dimers or polymers.
A typical example of fuel upgrading through dehydration reaction is dimethyl ether DME synthesis. A chemical reaction in which two or more molecules bond by losing one or more water molecules. Chemicals that deal with life.
We review their content and use your feedback to keep the quality high.
Dehydration Synthesis Definition Examples And Equations

The Mechanism Of Organolithium Reaction With Nitriles Organic Chemistry Organic Chemistry Study Chemistry Lessons

Dehydration Synthesis Definition Examples And Equations

Pin On Reactions Of Carboxylic Acids And Their Derivatives Practice Problems
No comments for "Dehydration Synthesis Reactions Are Used in Forming Which Compounds"
Post a Comment